This product is available solely through our 503A Compounding Pharmacy, ensuring personalized care and precision in every order. Please note that a valid prescription is required for purchase. If you do not have an account, please contact us.
Dosing is quite customized because the preparation is layered. Hypogonadal males usually begin treatment by having 1 mL (total 200 mg steroid content) administered profoundly into the gluteal muscle every seven to ten days offering a small 60 mg/week nandrolone anabolic adjuvant and approximating mid-physiologic testosterone exposure
Midway between injections, serum testosterone is drawn to guide titration; doctors usually seek the center of the reference range to prevent supra-physiological peaks.
Administration calls for a 22- or 23-gauge, 1- to 1.5-inch needle inserted at 90 degrees into properly relaxed muscle. Aspiration before injection proves there is no blood return. The vial is carefully heated in the hands to lower viscosity, swabbed with alcohol, and tapped with a fresh sterile needle for every draw to preserve sterility. Many patients would rather have clinic appointments until they have mastered the technique even if self-administration is possible following official education.
Baseline hematocrit and hemoglobin, three months, and then semi-annually comprise laboratory surveillance; annual fasting lipid panel; annual liver-function tests; and prostate-specific antigen plus digital Rectal examination following age-appropriate cancer-screening guidelines. If hematocrit surpasses 54 percent, PSA increases markedly, or LDL cholesterol rises despite lifestyle adjustments, reductions in dosage or interval lengthenings are required.
Treatment duration is determined by the indication. While patients using the combination for reversible catabolic conditions frequently taper once clinical objectives—weight restoration or hematologic correction—are achieved, lifelong replacement may be required in chronic primary hypogonadism.
To allow hypothalamic-pituitary axis recovery and to re-evaluate the ongoing need for anabolic support, doctors sometimes schedule drug holidays—eight to twelve weeks off medication.
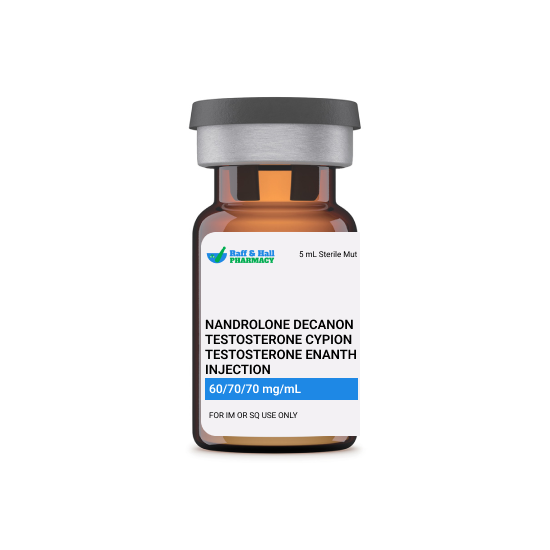
This combination injection contains nandrolone decanoate, testosterone cypionate, and testosterone enanthate—three anabolic and androgenic steroids used to treat conditions related to hormone deficiency, muscle wasting, or severe weight loss. It is prescribed for men with low testosterone levels and in cases where rebuilding muscle mass and strength is critical, such as after surgery, trauma, or chronic illness. Due to its powerful effects, this therapy is carefully managed by healthcare professionals and is often used as part of a broader treatment plan including lifestyle and nutritional support.
Each component plays a role in promoting growth and restoring hormonal balance. Nandrolone enhances protein synthesis and helps retain nitrogen in muscle tissues, supporting muscle repair and strength. Testosterone cypionate and enanthate work by replacing deficient testosterone, stimulating red blood cell production, improving bone density, and regulating fat distribution. Together, these compounds support anabolic processes, increase endurance, and boost energy by influencing metabolic pathways and hormone receptors throughout the body.
This medication should not be used in individuals with prostate or breast cancer, untreated heart or liver conditions, or severe kidney impairment. It is contraindicated in pregnant or breastfeeding women due to potential risks to the fetus or infant. People with a history of blood clots, sleep apnea, or high blood pressure should be monitored closely while on this treatment. Regular assessments including liver function, lipid levels, and hormone panels are necessary to ensure the patient’s safety during therapy.
These steroids may interact with blood thinners, insulin, corticosteroids, and other hormone-based therapies, possibly altering their effects or increasing risks. Using additional anabolic steroids or performance enhancers can intensify side effects such as liver strain or hormonal imbalance. Certain antidepressants, anti-hypertensive drugs, and diabetes medications should be reviewed by a healthcare provider prior to initiating therapy to avoid harmful interactions.
Common side effects include fluid retention, headaches, acne, and changes in mood or libido. In men, prolonged use may cause testicular shrinkage, breast enlargement, and fertility issues, while women may develop masculine features such as deepened voice or excess hair growth. Elevated cholesterol, liver enzyme changes, and high blood pressure are also possible. Rare but serious complications include cardiovascular events, liver toxicity, and psychological disturbances. Regular monitoring helps minimize risks and ensure that any adverse effects are addressed promptly.
This combination injection is not safe for use during pregnancy or while breastfeeding. Exposure to anabolic steroids during fetal development may result in birth defects, hormonal imbalances, or developmental delays. Similarly, these compounds may transfer through breast milk and affect the infant’s growth and hormonal health. Women who are pregnant, planning to become pregnant, or nursing should avoid this therapy and consult their healthcare provider to explore safer treatment alternatives.
Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
- Kishner, S., Morello, J.K., “Anabolic Steroid Use and Abuse”, Medscape: Endocrinology. October 2017. Retrieved from https://emedicine.medscape.com/article/128655-overview– LinkOpens in New Tab
- Patane, F.G., Liberto, A., Maglitto, A.N.M., Malandrino, P., Esposito, M., Amico, F., Cocimano, G., Li Rossi, G., Condorelli, D., Di Nunno, N., Montana, A., “Nandrolone Decanoate: Use, Abuse and Side Effects”, Medicina (Kaunas), vol. 56 issue 11. November 2020. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7696474/– LinkOpens in New Tab
- Geusens, P.,” Nandrolone decanoate: pharmacological properties and therapeutic use in osteoporosis”. Clinical Rheumatology suppl. 3, pp. 32-39. Available: https://pubmed.ncbi.nlm.nih.gov/8846659/#:~:text=A%20dose%20of%2050%20mg,patients%20with%20corticosteroid%20induced%20osteoporosis.– LinkOpens in New Tab
- Nandrolone Decanoate. Available: https://www.glowm.com/resources/glowm/cd/pages/drugs/n007.html.– LinkOpens in New Tab
- Nandrolone Decanoate, Drug Bank. Available: https://go.drugbank.com/drugs/DB08804– LinkOpens in New Tab
- Nandrolone Decanoate, PubChem. National Library of Medicine. Available: https://pubchem.ncbi.nlm.nih.gov/compound/Nandrolone-decanoate– LinkOpens in New Tab
- Nandrolone Decanoate Injection, USP. Available: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f059e04-1ebc-40bb-a49c-f9d90d41b1cb– LinkOpens in New Tab
- Aveed (testosterone undecanoate Injection) package insert. Malvern, PA: Endo Pharmaceuticals Solutions Inc.; 2014 Mar.
- DELATESTRYL (Testosterone Enanthate Injection, USP) package insert. Lexington, MA: Indevus Pharmaceuticals, Inc.; 2007 July.
- Axiron (testosterone) topical solution, package insert. Indianapolis, IN: Lilly USA, LLC; 2011 Dec.
- The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616-31.
- Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
- WD Finkle, S Greenland, GK Ridgeway, et al. Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men. DOI: 10.1371/journal.pone.0085805
- FDA Medwatch – FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. Retrieved January 31, 2014. Available on the World Wide Web https://www.fda.gov/Drugs/DrugSafety/ucm383904.htm– LinkOpens in New Tab
- Natesto (testosterone) nasal gel package insert. Durants, Christ Church Barbados: Trimel BioPharma SRL; 2014 May.
- Testim (testosterone gel) package insert. Malvern, PA: Auxilium Pharmaceuticals, Inc.; 2010 Apr.
- Androderm (testosterone transdermal system) package insert. Corona, CA: Watson Pharma, Inc.; 2014 Jun.
- Androgel (testosterone gel) package insert. Marietta, GA: Solvay Pharmaceuticals, Inc.; 2012 Sept.
- Striant (testosterone buccal system) package insert. Livingston, NJ: Columbia Laboratories, Inc.; 2014 Mar.
- Fortesta (testosterone) gel, package insert. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010 Dec.
- DEPO-TESTOSTERONE (testosterone cypionate) injection, package insert. New York, NY: Pharmacia & Upjohn Co.; 2006 Sept.
- Kochenour NK. Lactation suppression. Clin Obstet Gynecol. 1980;23:1045-1059.
- Nandrolone Pregnancy and Breastfeeding Warnings. Available: https://www.drugs.com/pregnancy/nandrolone.html.– LinkOpens in New Tab
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity, and partial androgen deficiency. Aging Male 2003;6:1—7.
- Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity, and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Clin Endocrinol 2006; 154:899—906.
- Hobbs CJ, Jones RE, Plymate SR. Nandrolone, a 19-nortestosterone, enhances insulin-independent glucose uptake in normal men. J Clin Endocrinol Metab 1996; 81:1582—5.
- Corrales JJ, Burgo RM, Garcia-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism 2004;53:666—72.
- Lee CH, Kuo SW, Hung YJ, et al. The effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocrine Res 2005;31:139—148.
- Goffin E, Pirson Y, Geubel A, et al. Cyclosporine-methyltestosterone interaction. Nephron 1991;59:174—5.
- Borras-Blasco J, Rosique-Robles JD, Peris-Marti J, et al. Possible cyclosporin-danazol interaction in a patient with aplastic anaemia. Am J Hematol 1999;62:63—4.
- Moller BB, Ekelund B. Toxicity of cyclosporine during treatment with androgens. N Engl J Med 1985;313:1416.
- Ross WB, Roberts D, Griffin PJ, et al. Cyclosporin interaction with danazol and norethisterone. Lancet 1986;1:330.
- Florinef® Acetate (fludrocortisone acetate) package insert. Bristol, TN: Monarch Pharmaceuticals; 2003 Jul.
- Humatrope™ (somatropin);package insert. Indianapolis, IN: Eli Lilly and Company; 2003 Jul.
- Zoladex® (goserelin acetate) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2003 Dec.
- Viadur® (leuprolide implant) package insert. Westhaven, CT: Bayer Pharmaceuticals; 2002 May.
- Aldercreutz H, Mazur W. Phyto-estrogens and western diseases. Annals of Medicine 1997;29:95—120.
- Krauser JA, Guengerich FP. Cytochrome P450 3A4-catalyzed testosterone 6beta-hydroxylation stereochemistry, kinetic deuterium isotope effects, and rate-limiting steps. J Biol Chem 2005;280:19496—506.
- Barnes KM, Dickstein B, Cutler GB Jr, et al. Steroid transport, accumulation, and antagonism of P-glycoprotein in multidrug-resistant cells. Biochemistry 1996;35:4820—7.
- Wells PS, Holbrook AM, Crowther NR et al. Interaction of warfarin with drugs and food. Ann Intern Med 1994;121:676—83.
- Androgel® (testosterone gel) package insert. Montrogue, France: Laboratories Besins International; 2005 Aug.
- Androderm® (testosterone transdermal system) package insert. Corona, CA: Watson Pharma, Inc.; 1999 Jan.
- Propecia® (finasteride) package insert. Whitehouse Station, NJ: Merck & Co., INC.; 2003 Oct.
- Avodart™ (dutasteride) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2005 May.
- Robbers JE, Tyler VE. Tyler’s Herbs of Choice: the Therapeutic Use of Phytomedicinals. Binghamton NY: Haworth Herbal Press, Inc.; 1999.
- German Commission E. Saw Palmetto berry, Sabal fructus, monograph Published March 2, 1989 and revised January 17, 1991. In: Blumenthal, M et al ., eds. The complete German Commission E Monographs -Therapeutic Guide to Alternative Medicines. Bosto
- Lazar JD, Wilner KD. Drug interactions with fluconazole. Rev Infect Dis 1990;12:S327—33.
- Hansten P, Horn J. The Top 100 Drug Interactions: A Guide to Patient Management. includes table of CYP450 and drug transporter substrates and modifiers (appendices). H & H Publications, LLP 2014 edition.
- Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity, and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Clin Endocrinol 2006; 154:899—90
- Corrales JJ, Burgo RM, Garcia-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism 2004;53:666—72
- Cohen JC, Hickman R. Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab 1987;64:960—3.
- Ranexa (ranolazine extended-release tablets) package insert. Foster City, CA: Gilead Sciences, Inc. 2013 Dec.
- Letairis™ (ambrisentan) package insert. Foster City, CA: Gilead Sciences, Inc; 2008 Oct.
- Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015 Jan.
- Gilotrif (afatinib) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2013 Nov.
- Androgel 1.62% (testosterone gel) package insert. North Chicago, IL: Abbott Laboratories; 2014 Nov.
- Naik BS, Shetty N, Maben EVS. Drug-induced taste disorders. European Journal of Internal Medicine 2010; 21:240-243.